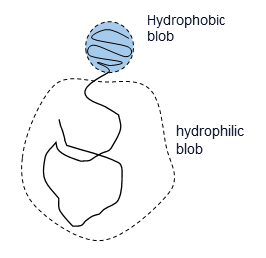
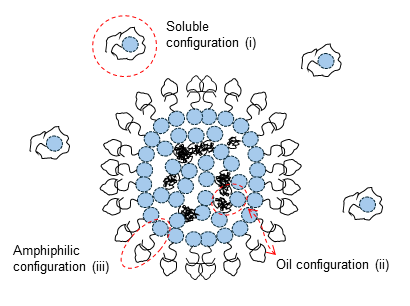
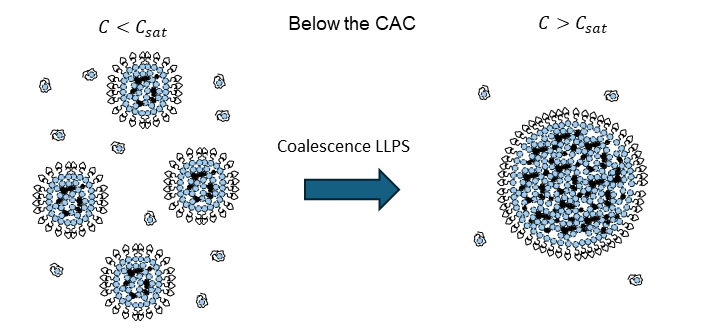
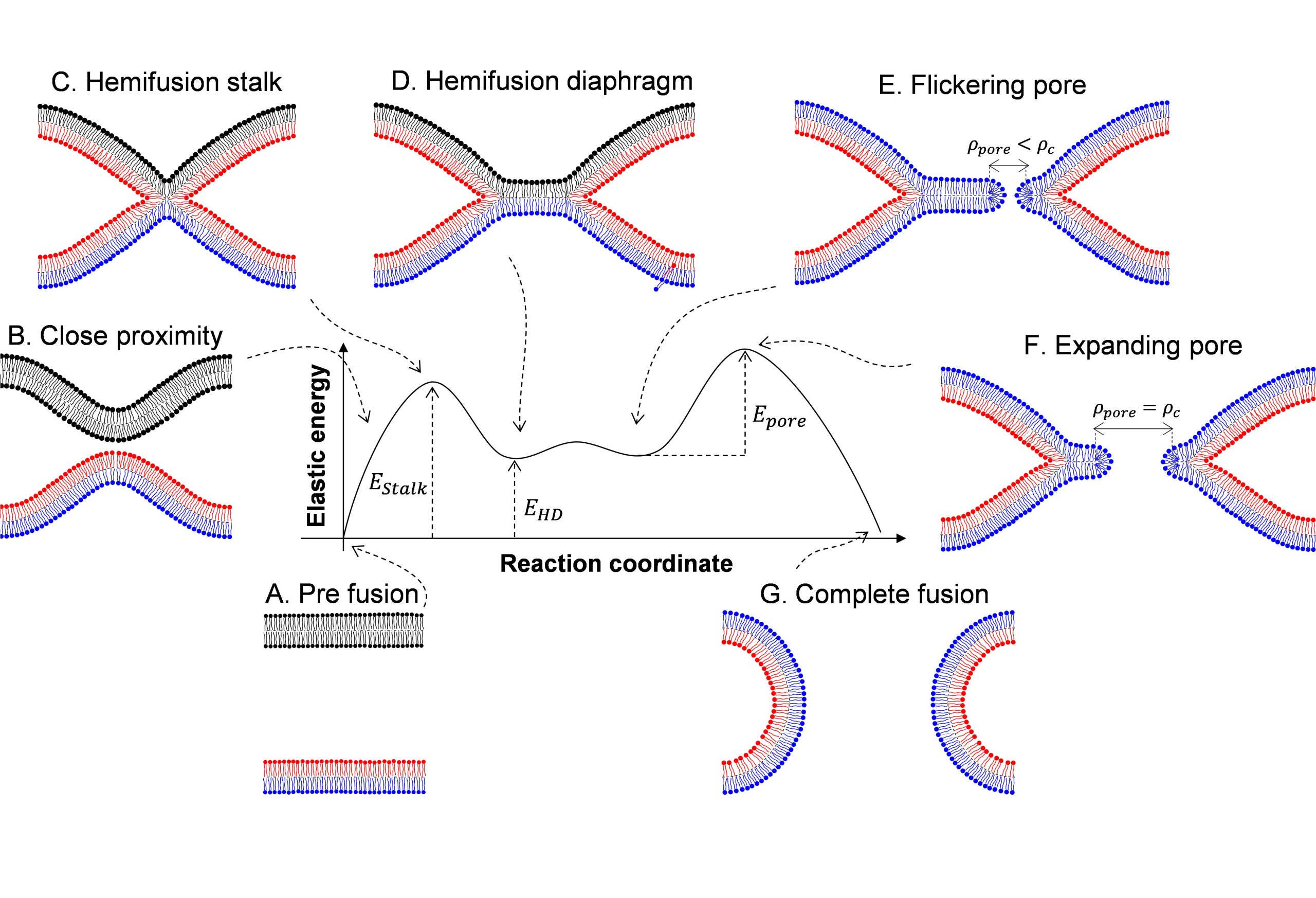
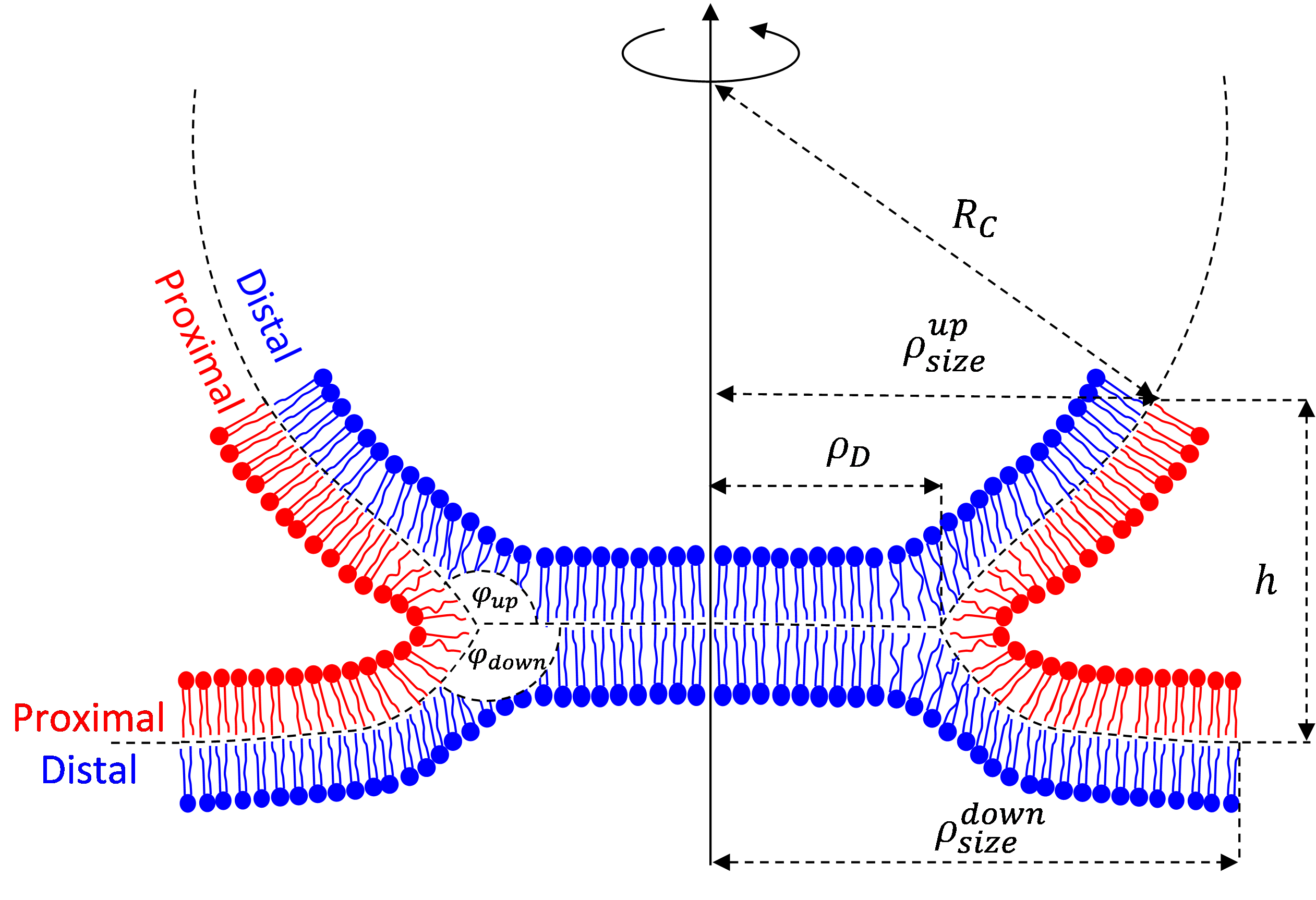
The physics of virus life cycle
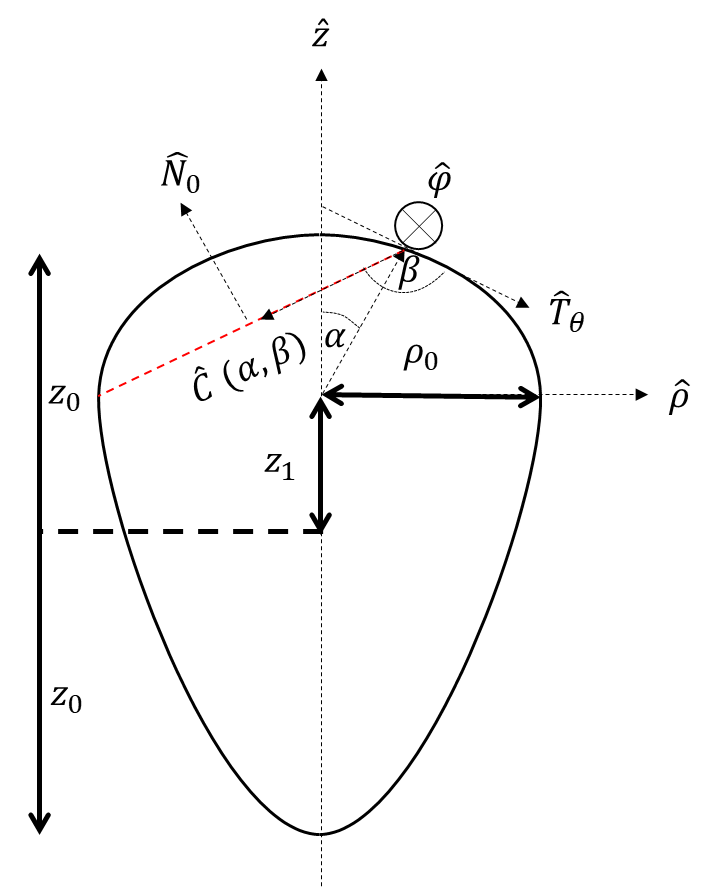
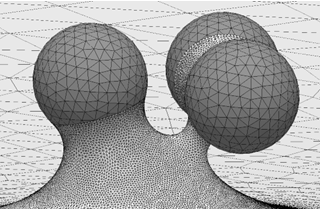
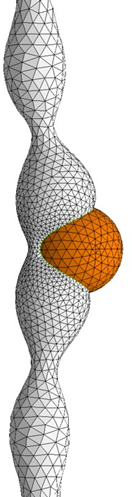
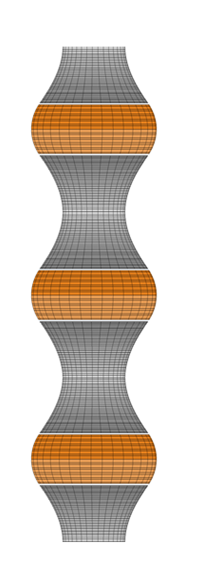
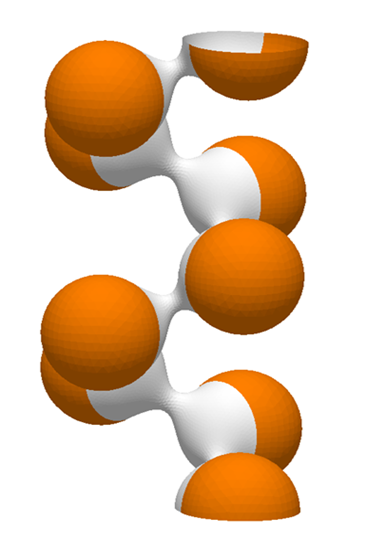
We focus on the mechanical and physical principles governing key stages of the viral life cycle: entry, replication, and egress. During entry, we study how viruses exploit cellular mechanics and membrane deformation to penetrate host cells. In the replication phase, we investigate how viruses restructure the cellular internal architecture and drive the formation of membrane-bound and membrane-less viral replication factories. For egress, we analyze the mechanical processes that allow viruses to overcome intracellular barriers and exit the host cell. By integrating these stages, we aim to uncover physical mechanisms that could inform the development of novel antiviral strategies.