Membrane-less Organelles Mechanics
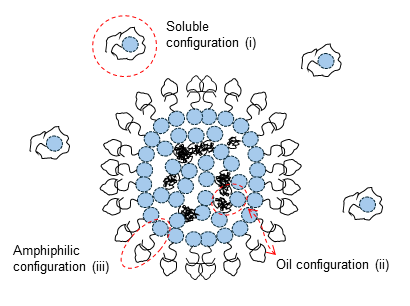
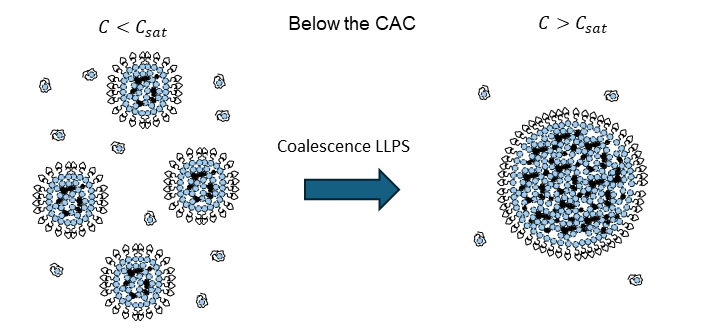
Membraneless organelles are biomolecular condensates formed through liquid-liquid phase separation of proteins and RNA or DNA molecules. These structures have emerged as key players in the spatial and functional organization of the cell interior and are integral to numerous biological processes, including RNA metabolism (transcription, splicing, and decay), DNA repair and replication, protein quality control and degradation, and signal transduction. By localizing specific biomolecules, these organelles enable the spatial and temporal regulation of cellular functions, enhance reaction rates, buffer cellular stress, and maintain homeostasis. Interestingly, several viruses exploit these mechanisms, forming membrane-less organelles that serve as viral replication factories, concentrating viral components for efficient assembly. The size and shape of these compartments are critical to their function. Our research focuses on understanding the mechanical properties of these organelles and developing theoretical and computational frameworks to predict their size distributions and shape deformations, particularly as virions form within them.
Fusion (viral entry) and fission (viral release) are critical steps in the enveloped virus life cycle. As a result, both viruses and host cells have evolved opposing mechanisms to control the magnitude of these energy barriers to suit their needs. Viruses have developed strategies to lower the energy barriers, facilitating their entry into cells via the plasma and endosomal membranes. In contrast, cells have evolved mechanisms to increase these energy barriers, strengthening their defenses against viral invasion. Our interest is to use physical modeling and computer simulations to better understand this tug-of-war between host and pathogen.